ACHONDROPLASIA
Brief Summary
Achondroplasia, also known as Achondroplastic dwarfism and ACH, is a congenital disorder of the bones particularly in the arms and legs characterized by dwarfism in most cases. Though not the cause of all dwarfism cases, the disease is synonymous with the conditions as 70% of those suffering from dwarfism are due to Achondroplasia. Due to the growth disorder, this disease causes developmental issues that can increase the mortality of those afflicted. There is no known treatment to the disease and symptoms can only be managed through lifelong medication and stringent care.
Frequency
It is the most common type of short-limbed dwarfism; occurs in 1 in 15,000 to 40,000 newborns.
Inheritance
Achondroplasia is inherited as an autosomal dominant with essentially complete penetrance.
History of Disease
Achondroplasia has been recorded throughout human history over many different cultures and locations. However, babies born with this condition were often left to die or killed due to cultural stigmas and supernatural fears over the condition. The earliest archaeological evidence for the disease were skeletal remains from a person with the condition in ancient Egypt in 4500 BCE. Those suffering from this disease have often been ostracized throughout history either persecuted or used as amusement and entertainment. Only in 1994 did we understand the genetic cause of this disease by the research of Dr. John Wasmuth.


Pathogenesis
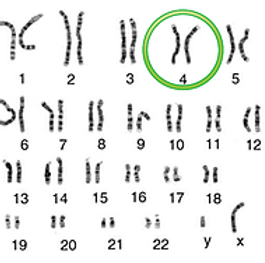
Achondroplasia is caused by a mutation in the FGF3 gene in chromosome 4 at 4p 16.3. This mutation causes a decreased production of fibroblast growth factor receptor 3 which is crucial in the conversion of cartilage into bone which is vital in the proper development of a person. This mutation is inherited in an autosomal dominant manner. More than 80 percent of people with achondroplasia have parents with normal characteristics and are born with achondroplasia due to a recent (de novo) gene modification (mutation).
Pathogenesis
Achondroplasia is caused by a mutation in the FGF3 gene in chromosome 4 at 4p16.3. This mutation causes a decreased production of fibroblast growth factor receptor 3 which is crucial in the conversion of cartilage into bone. This is vital in the proper development of the person. This mutation is inherited in an autosomal dominant manner. More than 80 percent of people with achondroplasia have parents with normal characteristics and are born with achondroplasia due to a recent (de novo) gene modification (mutation).
Symptoms
1
slender trunk
2
short extremities, particularly in the proximal (rhizomelic) parts
3
a wide frontal bossed head
4
midface hypoplasia
5
trident hand configuration
6
hyperextensibility of most joints, particularly the knees, is normal, but the elbow has restricted extension and rotation
7
a thoracolumbar gibbus is usually present at birth, but as the infant starts to ambulate, it typically gives way to exaggerated lumbar lordosis; mild to moderate hypotonia is common, and motor milestones are usually delayed; until hydrocephalus or other central nervous system problems occur, knowledge
is natural
8
higher infant mortality rate but have a relatively normal adult life expectancy
Social Concerns
Achondroplasia is one of the oldest and most recognizable congenital disorders. Unlike some other genetic disorders, the symptoms of Achondroplasia are evident at birth and throughout the life of those affected. Because of the very visible physical differences, people suffering from Achondroplasia face many issues in society as they are often subject to mockery, social ridicule, and negative biases towards them. They are also more at risk of developing mental disorders such as depression and anxiety disorder, not due to their disorder, but because of the increased likelihood of the person facing social ostracization and bullying in childhood as the disorder causes no decrease in mental faculties. The potential psychological damage these people experience are due to societal and social factors.
Treatment
Normally, due to the very pronounced and evident features presented by the patient a person can be easily identified with this disease at birth by clinical prognosis, however to 100% guarantee the condition and the specific cause of the dwarfism to be Achondroplasia. A diagnosis is done based on the typical clinical and radiologic features; the delineation from severe hypochondroplasia may be arbitrary. A basic approach for prenatal diagnosis of ACH homozygotes in families at risk and in which parents are heterozygous for either the 1138A or 1138C allele is the demonstration of a very small range of mutations triggering Achondroplasia and the ease with which they can be identified (1 PCR and 1 restriction digest). Due to the nature of Achondroplasia, there is no known cure for the disease, however there are treatments to manage or possibly reduce the severity of symptoms during the developmental years of childhood. Treatment with injections of a synthetic version of the hormone may increase final height for people with dwarfism due to growth hormone deficiency. In most cases, infants are given regular shots for several years before they reach their full adult height, even within their family's average adult range.
Clinical Trials
Title:
A Dose Escalation Trial Evaluating Safety, Efficacy, and Pharmacokinetics of Multiple Subcutaneous Doses of TransCon CNP Administered Once Weekly in Children With Achondroplasia
Intervention:
Drug: TransCon CNP
TransCon CNP drug product is a lyophilized powder in a single-use vial containing either TransCon CNP 3.9 mg CNP-38/vial or TransCon CNP 0.80 mg CNP-38/vial. Prior to use, the lyophilized powder is reconstituted with sterile water for injection and administered by subcutaneous injection via syringe and needle.
Drug: Placebo for TransCon CNP
Weekly subcutaneously injection of placebo.
Phase:
Phase 2
Procedure:
TransCon CNP is an investigational (new) drug, which means that it is currently being tested, and therefore is considered experimental. TransCon CNP is designed to provide a sustained exposure of active CNP by subcutaneous (under the skin) injection once weekly.
The Randomized Period of this study is a double-blinded and placebo-controlled. "Placebo-controlled" means that some participants will receive injections that don't contain any TransCon CNP (placebo injection - no active ingredient). "Double-blinded" means that neither the participant nor the study doctor will know which treatment the participant will be receiving, except in an emergency.
After completion of the Randomized Period the trial participant may be invited to take part of the Open-Label Period of this study.
"Open-label" means that all participants will receive injections that contain TransCon CNP; regardless of which treatment (TransCon CNP or placebo) was assigned during the 52 weeks (1 year) Randomized Period/blinded treatment period. It also means that both the participant and the study doctor will know which treatment, and which dose the participant receives.
Goal:
Experimental: TransCon CNP 50 mcg
TransCon CNP 50 mcg CNP/kg or placebo mimicking TransCon CNP 50 mcg delivered once weekly by subcutaneous injection
Interventions:
Drug: TransCon CNP
Drug: Placebo for TransCon CNP
Experimental: TransCon CNP 100 mcg
TransCon CNP 100 mcg CNP/kg or placebo mimicking TransCon CNP 100 mcg delivered once weekly by subcutaneous injection
Interventions:
Drug: TransCon CNP
Drug: Placebo for TransCon CNP
Experimental: TransCon CNP >100 mcg
TransCon CNP >100 mcg CNP/kg delivered once weekly by subcutaneous injection (to be determined after completion of 100 mcg cohort)
Interventions:
Drug: TransCon CNP
Drug: Placebo for TransCon CNP
Further details at:
Expert Directory
References
[2]Achondroplasia. (n.d.) omim entry #100800. Retrieved from https://omim.org/entry/100800
[4]Fernandez, A. et al. (2009). Social and Educational Dimension. fundacionalpe.org
[5]FGFR3 gene. (2020). MedlinePlus. Retrieved from https://medlineplus.gov/genetics/gene/fgfr3/
FOR INFORMATION
FOR IMAGES